Pioneering Regenerative Medicine Drug Therapies for Heart Disease
Heart Disease is the Leading Cause of Death Worldwide
Revidia Therapeutics is developing first-in-class drug therapies to repair and regenerate damaged heart tissue, offering new hope for patients with heart failure and other serious cardiac conditions.
Explore our innovative approach to cardiac regenerative medicine and learn how Revidia is addressing the fatal cardiac complications of Duchenne muscular dystrophy (DMD) and other types of heart disease and injury.
SCIENCE
Beyond Skeletal Muscle Gene Therapies for Duchenne Muscular Dystrophy
Duchenne muscular dystrophy (DMD) is a rare and fatal muscle wasting disease caused by mutations in dystrophin.
Gene therapies in development for DMD aim to partially restore dystrophin function in skeletal muscle and reduce symptoms of skeletal muscle wasting.

Revidia Therapeutics is Addressing the Main Cause of Mortality in DMD Patients – Heart Failure
The complex structure and function of the heart present unique obstacles to the development of effective gene therapies for heart failure
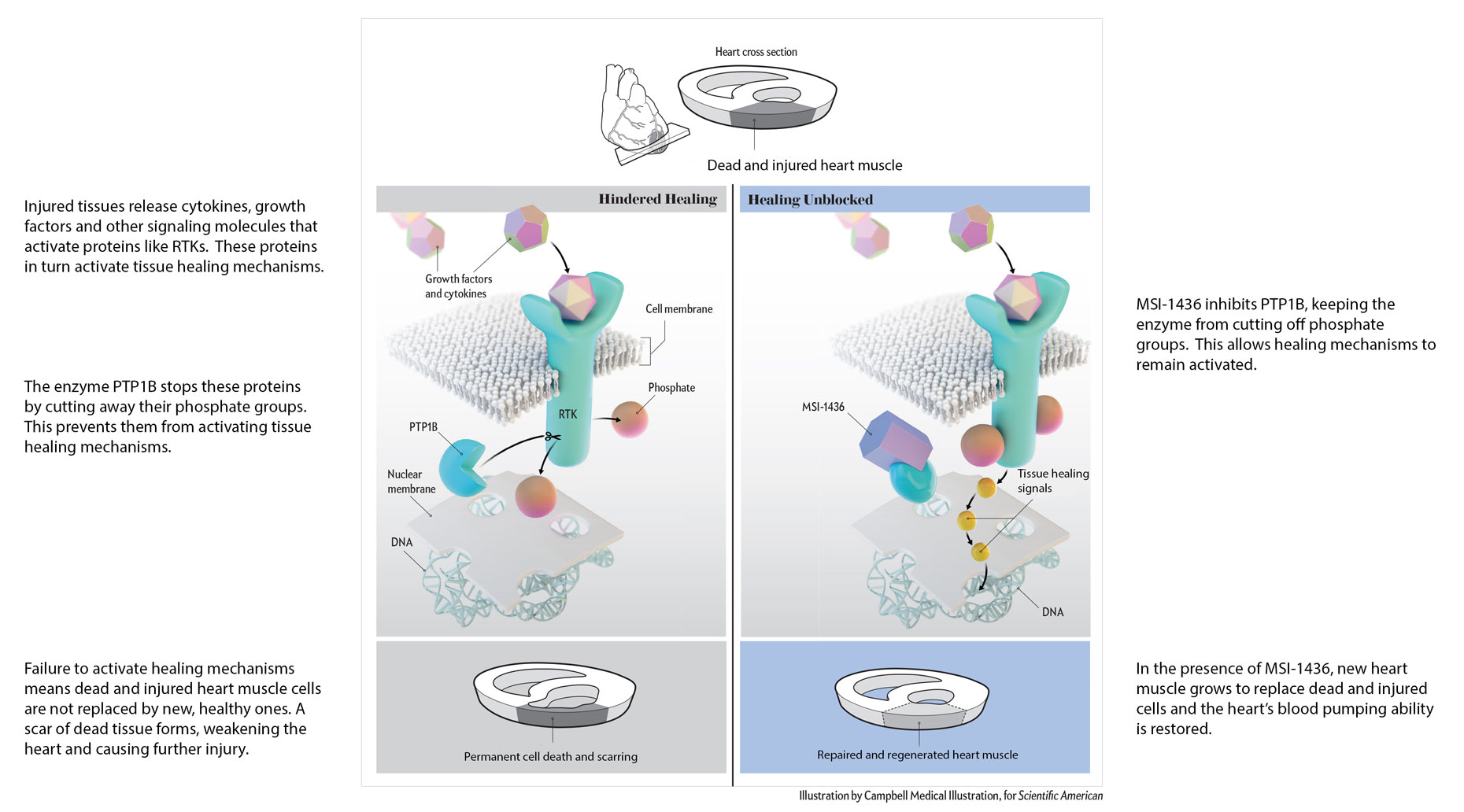
Revidia’s first-in-class drug candidate, MSI-1436, reactivates the heart’s natural repair and regenerative capacity by inhibiting a protein called PTP1B.

PTP1B acts as a “brake” on the heart’s natural repair mechanisms. By releasing this brake, MSI-1436 stimulates the repair and regeneration of damaged heart muscle and improves heart function.
Addressing the Unmet Need in DMD Cardiomyopathy: MSI-1436 Development Timeline
Efficacy
- Demonstrated efficacy in 3 animal species and 6 types of cardiac injury
- Demonstrated efficacy in multiple non-cardiac tissues
- Novel formulations and dosing strategies established
Safety
- FDA required safety testing complete
- Drug manufacturing methods established
- Previously established human safety
Clinical Trials
- FDA pre-IND meeting complete
- Phase 1/2a clinical trial design in DMD patients in progress
- IND application filing in progress
- Clinical trial fundraising in progress
- Protected by 4 U.S. and foreign patents and FDA Orphan Drug Designation; 3 additional patent applications pending
ABOUT
A Different Approach, a Shared Goal: Improving the Lives of Heart Disease Patients
Mission
Revidia Therapeutics was founded to address the limitations of current cardiac regenerative medicine R&D. Our paradigm shifting approach utilizes novel animal models to identify drug candidates with the ability to reactivate the innate regenerative capacity in damaged heart muscle.
Team
We have assembled a team of experts dedicated to challenging the status quo in the treatment of heart injury. Our scientists, clinicians, and drug developers bring a wealth of experience and a shared passion for improving the lives of heart disease patients.
Values
We are driven by a commitment to scientific excellence, patient-centricity, and a belief in the power of innovation to transform lives.
Leadership Team

Kevin Strange, Ph.D.
Founder and CEO
Kevin Strange, Ph.D.
Founder and Chief Executive Officer
Dr. Kevin Strange is an internationally recognized biomedical scientist, and entrepreneur. His research has been funded for more than 35 years by the National Institutes of Health, Department of Defense, and private foundations, and has been published in leading scientific journals including Science, PNAS, Neuron and Cell. He is the recipient of numerous awards including the John C. Parker Endowed Chair and Pittinger Award for Excellence in Basic Research. Dr. Strange earrned his Ph.D. from the University of British Columbia and conducted postdoctoral training in membrane and renal physiology at the National Institutes of Health.
After a distinguished scientific and leadership career at Harvard Medical School and Vanderbilt University School of Medicine, Dr. Strange became the first president of the MDI Biological Laboratory in 2009 where he established the Kathryn W. Davis Center for Regenerative Biology and Medicine. The Davis Center was highlighted twice in the U.S. Congressional Record for its scientific accomplishments including the discovery of regenerative medicine drug candidates. Dr. Strange founded Revidia Therapeutics in 2013 to develop MSI-1436 and other small molecule regenerative medicine therapeutics.

Michael Christensen, Ph.D.
Executive Vice President for Business Development
Michael Christensen, Ph.D.
Executive Vice President for Business Development
Dr. Michael Christensen has spent more than 20 years in the pharmaceutical industry and has helped bring more than a dozen drugs to market, including Ozempic® (semaglutide), Tresiba® (insulin degludec), Isturisa® (osilodrostat), Recorlev® (levoketoconazole), Micardis® (telmisartan), and other drug products in the cardiometabolic space. He earned his Ph.D. in pharmacology at Vanderbilt University and completed his postdoctoral training in immunology at the Genomics Institute of the Novartis Research Foundation (GNF).
Dr. Christensen’s experience at both large and small pharmaceutical companies includes clinical trial development and execution, regulatory and medical affairs, and business and marketing operations. He has worked extensively with regulatory bodies including the FDA, Medicare/Medicaid and Veterans Affairs. In addition to his role at Revidia, Dr. Christensen is also the President and Chairman of the Board of Directors at Prometheus Cardiology and Rare Disease, Inc.

Michael Stein, M.D., F.A.C.C.
Vice President for Clinical Development
Michael Stein, M.D., F.A.C.C.
Vice President for Clinical Development
Dr. Michael Stein is a cardiologist, well-published clinician scientist and experienced drug developer. He previously served as Vice President and Head of Study Medical Experts for Bayer Pharmaceuticals; Senior Director and Project Head of Clinical Development for Ikaria Biopharmaceuticals; Senior Director for Clinical Development at Novo Nordisk Pharmaceuticals; Senior Medical Director for Daiichi Sankyo; and Director of Cardiovascular Clinical Development at AstraZeneca. Dr. Stein has played key roles in bringing diverse therapies drugs to clinical practice including AZD6140/ticagrelor/Brilinta® and Prasugrel/Effient® for reducing clot formation in heart attack and stroke patients; Anti-TFPI antibody for improving coagulation in hemophilia patients; and IK5001 hydrogel for reduction or elimination of ventricular remodeling following large myocardial infarction.
Dr. Stein received his M.D. from the University of Illinois and fellowship training in cardiology and interventional cardiology at the University of Iowa. He has served on the medical faculties at the of Wisconsin College of Medicine and Emory University College of Medicine. His basic research efforts have focused on diverse aspects of cardiovascular physiology and pathophysiology.
Scientific Advisory Board

Richard T. Lee, M.D.
Richard T. Lee, M.D.
Dr. Richard Lee is a specialist in cardiovascular medicine and director of the Regenerative Medicine Center at Brigham and Women’s Hospital. He is also professor of medicine at Harvard Medical School and professor of Stem Cell and Regenerative Biology at Harvard University. His research focuses on developing new approaches for treating cardiovascular and metabolic diseases using genomics, stem cell biology, and molecular biology. Dr. Lee has published over 230 peer-reviewed articles based on his research and teaches undergraduates at Harvard College. He is the recipient of the prestigious National Institutes of Health Director’s Award.

Thomas A. Rando, M.D., Ph.D.
Thomas A. Rando, M.D., Ph.D.
Dr. Tom Rando is director of the UCLA Broad Stem Cell Research Center. His research focuses on muscle stem cell biology, with particular interests in stem cell aging and stem cell therapeutics. Dr. Rando pioneered studies using muscle stem cells to replace or restore functional muscle in preclinical studies of trauma and degenerative diseases such as muscular dystrophies. He has received numerous awards including the NIH Director’s Pioneer Award, the “Breakthroughs in Gerontology” Award from the American Federation for Aging Research, and a Transformative Research Award from the NIH.
Medical Advisory Board

Linda Cripe, M.D.
Linda Cripe, M.D.
Dr. Linda Cripe is professor of pediatrics and a pediatric cardiologist for The Heart Center at Nationwide Children’s Hospital. She is also a member of the physician team for the Neuromuscular Disorders section of the Nationwide Neurosciences Center. Dr. Cripe’s clinical interests focus on non-invasive cardiac imaging specifically echocardiography as well as on the care and treatment of cardiomyopathy associated with neuromuscular disease, such as Duchenne muscular dystrophy (DMD). She was a member of the Centers for Disease Control National Steering Committee Duchenne Muscular Dystrophy Standards of Care and has been an invited lecturer nationally and internationally on cardiomyopathy related to DMD. Dr. Cripe is currently a member of the Scientific Advisory Board for Parent Project Muscular Dystrophy (PPMD).

Jennifer Monti, M.D., M.P.H.
Dr. Jennifer Monti is a board-certified cardiologist subspecialized in cardiogenetic disorders of young adults. She received her undergraduate degree in biochemistry from Harvard College and degrees in medicine and public health from Case Western Reserve University, with residency and fellowship training at Johns Hopkins and the University of Pennsylvania. Dr. Monti’s research and clinical expertise include heart failure, vascular biology, sudden cardiac death, and public-private investments in emerging technologies and business development. She is the founder and CEO of Shock Analytics, on the health team @Meta, and an entrepreneur in residence at Northeastern University’s Roux Institute.
NEWS

Revidia Therapeutics Expands Leadership Team
Also awarded a small business innovation research grant from the National Heart, Lung, and Blood Institute.
Revidia Therapeutics Executive Summary
CONTACT
If you are interested in learning more about Revidia’s programs and investment and partnership opportunities, please contact us at: